Sodium Alkali Spray Desulfurizer Tower Flue Gas Purification SO2
- Category: Desulfurization tower
- Application: flue gas purification
- Desulfurization efficiency:>92%
- Permitted fluctuation of flue gas: 30%-110%
- Cylinder resistance:900-1100 Pa
Sodium Alkali Spray Desulfurizer Tower Flue Gas Purification SO2
1. Brief introduction
Sodium Alkali Spray desulfurizer tower is widely applied in kiln flue gas purification in brick and tile industry. It uses alkali as solvent and could absorb acid gases like SO2, So3, HCl, etc.
The sodium alkali desulfurization process mainly consists of SO2 absorption system, absorbent system, and other components. The SO2 absorption system consists of a desulfurization tower, an internal spray system, a cyclone, a mist eliminator, an absorption liquid circulation pump, a supply pipeline, and other components.
2. Main technical parameters
|
Desulfurization efficiency |
≥92%, (pH>9) |
|
Allowable fluctuation range of flue gas volume |
30%~110% |
|
Cylinder resistance |
900~1100Pa |
3. The working principle
This method uses NaOH solvent to absorb the SO2 in the flue gas, the reaction equation as below:
2NaOH+SO2→Na2SO3+H2O (1)
Na2SO3+H2O+SO2→2NaHSO3 (2)
Notes:
a. Equation (1) represents the main reaction of SO2 absorption by the desulfurization solution during the start-up stage, when the NaOH solution absorbs SO2 and the pH value of the regeneration solution is high (above 8);
b. Equation (2) represents the main reaction when the pH value of the desulfurization solution is low (5-8).
c. As the absorption process going, the amount of Na2SO3 in the absorption solution increases, and the absorption capacity of the absorption solution decreases. It is necessary to add alkaline solution to the absorption solution, maintain a relatively stable concentration ratio of Na2SO3 in the absorption solution, and regularly discharge a portion of the waste liquid.
d. The acidic gases such as SO3 and HCl contained in the flue gas will also be absorbed by the absorbent.
4. The structure diagram of desulfurizer tower
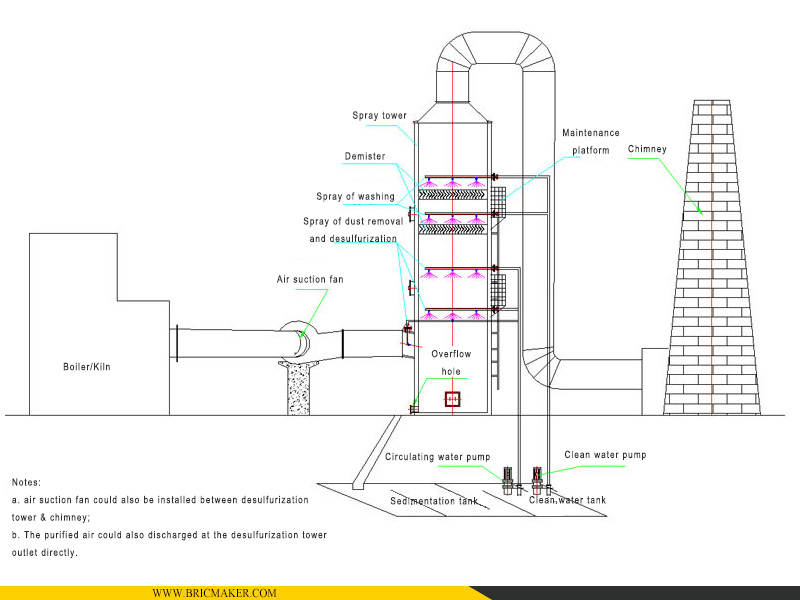
5. Detail images:


INQUIRY
CATEGORIES
- Automatic Clay Brick Production Lines
- Rotary Tunnel Kiln
- Tunnel Kilns
- Vacuum Extruder
- Green Brick Cutting Group Transfer System
- Brick's Robot Auto Stacking System
- Brick's Raw Material Process Machines
- Aging Warehouse Equipment
- Red bricks load & package machine
- Kilns' Refractory Materials
- Desulfurization tower
CONTACT US
Name: General Manager
Mobile:+8613151630928
Whatsapp:+8613222235952
Email:sales@bricmaker.com
Add:No.1, Huayu Road, Donglai Development Zone, Zhangjiagang City, Jiangsu, China







