
Пылеуловитель и распылительная десульфуризационная башня для очистки SO₂
1. Краткое описание
Башня для очистки SO₂ широко используется для газоочистки в кирпичной и черепичной промышленности. В качестве растворителя применяется щёлочь, способная поглощать кислотные газы, такие как SO₂, SO₃, HCl и др.
Дымовые газы из печи поступают в десульфуризационную башню по тангенциальной траектории. Мелкие частицы дыма и пыли, содержащиеся в газе, смачиваются и сталкиваются между собой, образуя более крупные частицы. Под действием центробежной силы они отбрасываются к стенкам башни и, благодаря водяной плёнке, смываются к её основанию. Затем дымовые газы закручиваются вверх вдоль стенки башни и на высокой скорости сталкиваются с распылённой десульфуризационной жидкостью во вращающемся слое. В этот момент происходит полный контакт трёх фаз — газовой, жидкой и твёрдой, — и содержащийся в выхлопных газах SO₂ в основном поглощается щёлочными компонентами жидкости, тем самым полностью очищая газы. Очищенные дымовые газы проходят обезвоживание через каплеуловители и отводятся через гибкую дымовую трубу.
Оставшийся после десульфуризации раствор сливается из нижней части башни в канал для промывки золы. После отстаивания в отстойнике (с небольшим количеством пыли из дымовых газов) осветлённая жидкость с помощью насоса снова подаётся в башню и используется повторно. Небольшое количество сточных вод сбрасывается из установки десульфуризации. Через определённый промежуток времени осадок в циркуляционном резервуаре удаляется вручную или механически.
2. Основные технические параметры
| Эффективность десульфуризации |
≥92%, (pH>9) |
|
Допустимый диапазон колебаний объёма дымовых газов |
30%~110% |
|
Сопротивление цилиндра |
900~1100Pa |
3. Преимущества щелочной десульфуризационной башни
Система отличается низким соотношением жидкость/газ и низким энергопотреблением при эксплуатации. В качестве поглотителя десульфуризации используется натрий-щелочной раствор, обеспечивающий высокую эффективность очистки — как правило, выше 90%.
Благодаря использованию технологии завихряющей струи и давления при распылении, сопротивление оборудования незначительно.
При натрий-щелочной десульфуризации циркулирующая вода представляет собой водный раствор, содержащий ионы Na⁺. В процессе циркуляции отсутствует коррозия или засорение насосов, трубопроводов и оборудования. Работа системы стабильна и надёжна, а эксплуатация и обслуживание оборудования удобны.
Регенерация абсорбента и осаждение десульфуризационного шлама происходят вне башни, что предотвращает засоры и износ внутри башни, повышает надёжность работы и снижает эксплуатационные расходы.
При изменении типа топлива и нагрузки котла возможно корректировать значение pH, соотношение жидкость-газ и другие параметры системы для обеспечения требуемой эффективности десульфуризации.
Процесс простой и не склонен к образованию накипи. Система не сбрасывает сточные воды от десульфуризации и не создаёт вторичного загрязнения. Особенно хорошо подходит для промышленных котлов и печей.
4. Принцип работы
Десульфуризационная башня использует раствор NaOH для поглощения SO₂ из дымовых газов. Уравнения реакций приведены ниже:
2NaOH + SO₂ → Na₂SO₃ + H₂O
Na₂SO₃ + H₂O + SO₂ → 2NaHSO₃
Примечания:
a. Уравнение (1) отражает основную реакцию поглощения SO₂ десульфуризационным раствором на стадии запуска, когда раствор NaOH поглощает SO₂, а значение pH высокое (выше 8);
b. Уравнение (2) описывает основную реакцию при пониженном pH десульфуризационного раствора (5–8);
c. По мере протекания процесса поглощения концентрация Na₂SO₃ в растворе увеличивается, что снижает его поглощающую способность. Необходимо регулярно добавлять щелочной раствор для поддержания стабильного соотношения Na₂SO₃, а также периодически удалять часть отработанного раствора;
d. Кислотные газы, такие как SO₃ и HCl, также эффективно поглощаются абсорбентом.
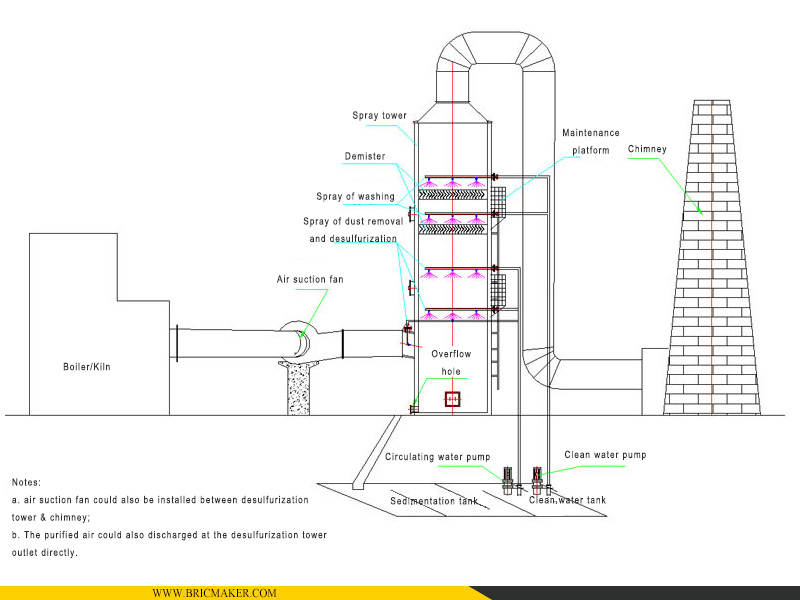
5. Схема конструкции десульфуризационной башни
Система поглощения SO₂ включает в себя:
десульфуризационную башню, внутреннюю систему распыления, циклон, каплеуловитель, насос циркуляции абсорбента, силовой трубопровод и другие компоненты.
6. Detail images:

Имя: General Manager
мобильный:+8613151630928
Тел.:+8613222235952
Whatsapp:+8613222235952
Почта:sales@bricmaker.com
Добавлять: Китай, провинция Цзянсу, город Чжанцзяган, улица Восточная Лай Хуаю, дом 1.
We chat
