
Десульфуризационная башня распылительного типа для очистки дымовых газов
Краткое описание
Десульфуризационная башня для очистки дымовых газов широко применяется в кирпичной и черепичной промышленности для очистки газов. В качестве растворителя используется щёлочь, которая способна поглощать кислотные газы, такие как SO₂, SO₃, HCl и другие.
Дымовые газы из печи поступают в десульфуризационную башню тангенциально, мелкие частицы дыма и пыли в газах смачиваются и сталкиваются друг с другом, образуя более крупные частицы. Под действием центробежной силы они оседают на стенки башни и смываются вниз под тонкой пленкой воды. Затем дымовые газы вращаются вверх вдоль стенок башни и сталкиваются с распылённым десульфуризационным раствором на высокой скорости через вихревой слой. В этот момент происходит интенсивный контакт газообразной, жидкой и твёрдой фаз — щёлочные компоненты раствора эффективно поглощают SO₂, обеспечивая полную очистку дымовых газов. Очищенные газы обезвоживаются уловителем тумана и выводятся через дымовую трубу, соответствуя нормам.
Отработанный раствор после десульфуризации выводится из нижней части башни в канал для промывки золы. После осаждения в отстойнике (в котором остаётся небольшое количество пыли) осветлённый раствор снова подаётся насосом в башню для повторного использования. Небольшое количество сточных вод удаляется из системы десульфуризации; периодически осадок из циркуляционного бассейна очищается вручную или механически.
Основные технические параметры
|
Эффективность десульфуризации |
≥92%, (pH>9) |
|
Допустимый диапазон колебаний объёма дымовых газов |
30%~110% |
|
Сопротивление цилиндра
|
С900~1100Pa |
3. Принцип работы
Данный метод использует раствор NaOH для поглощения SO₂ из дымовых газов. Уравнения реакций приведены ниже:
2NaOH + SO₂ → Na₂SO₃ + H₂O
Na₂SO₃ + H₂O + SO₂ → 2NaHSO₃
Примечания:
a. Уравнение (1) отражает основную реакцию поглощения SO₂ десульфуризационным раствором на стадии запуска, когда раствор NaOH поглощает SO₂ и значение pH регенерирующего раствора высокое (выше 8);
b. Уравнение (2) отражает основную реакцию при более низком значении pH раствора десульфуризации (5–8);
c. По мере протекания процесса поглощения количество Na₂SO₃ в растворе увеличивается, а его поглощающая способность снижается. Необходимо добавлять щелочной раствор в абсорбент, поддерживая относительно стабильное соотношение концентрации Na₂SO₃ в растворе, а также регулярно удалять часть отработанного раствора;
d. Кислотные газы, такие как SO₃ и HCl, содержащиеся в дымовых газах, также поглощаются абсорбентом.
4. Схема конструкции десульфуризационной башни
Система поглощения SO₂ состоит из десульфуризационной башни, внутренней системы распыления, циклона, уловителя тумана, насоса циркуляции абсорбционной жидкости, трубопровода подачи и других компонентов.
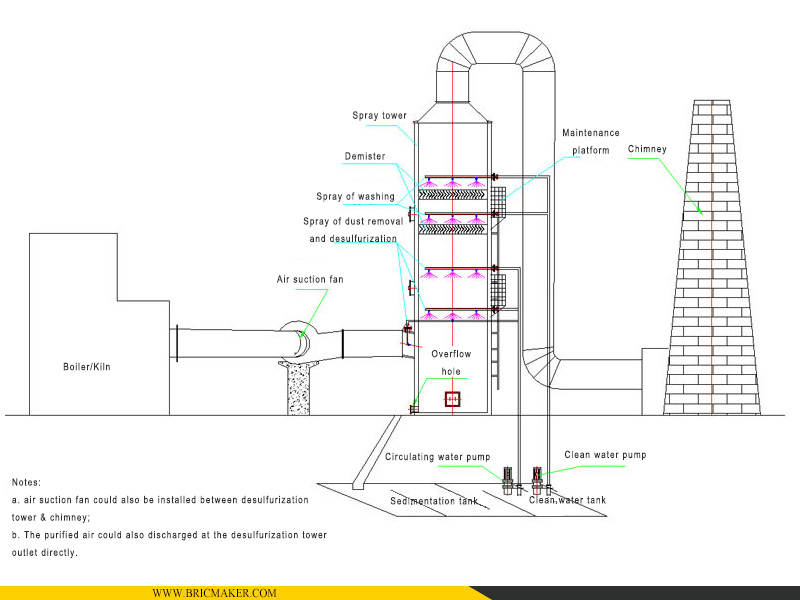
5. Detail images:


Имя: General Manager
мобильный:+8613151630928
Тел.:+8613222235952
Whatsapp:+8613222235952
Почта:sales@bricmaker.com
Добавлять: Китай, провинция Цзянсу, город Чжанцзяган, улица Восточная Лай Хуаю, дом 1.
We chat
